Neuronal aggregates of αSyn (Lewy bodies and neurites) are a pathological hallmark of Parkinson’s disease (PD). Moreover, genetic increases in αSyn expression and point mutations in the αSyn gene are an increasingly well-documented precipitant of early-onset familial PD (fPD). We recently discovered that αSyn, a neuronal protein of unclear physiological function, normally exists in cells and brain tissue principally as a α-helically folded tetramer that resists pathological aggregation. New preliminary experiments indicate that the tetrameric form is not only resistant to time-dependent self-aggregation but also shows increased resistance (compared to unfolded monomers) to misfolding initiated by tiny amounts of fibrillar material, i.e., “seeded aggregation”. Based on our new findings, it is important to identify factors that could trigger the denaturation of folded αSyn and allow its abnormal aggregation in neurons. Our unexpected discovery suggests that we should reconsider the common views that oligomeric αSyn is necessarily abnormal and pathogenic and that normal αSyn is ‘natively unfolded’, and it also implies that excess levels of free monomers represent a potentially harmful situation. Thus, we hypothesize that destabilization of the physiological tetramer through disease-causing familial mutations or certain environmental factors can lead to partially unfolded monomeric αSyn preceding the initiation of pathologic aggregation into cytotoxic amyloid oligomers. This is situation is analogous to thranthyretin-based cardiac and neural amyloidosis. We postulate that equilibrium shifts between native and abnormal αSyn in neurons and extracellular fluids could serve as an early marker of disease progression.
Lab projects currently focus on four major aspects:
– Mechanistically determine how stability of native tetrameric αSyn is compromised by factors associated with increased risk for PD.
– Diagnostically validate the shift in the αSyn tetramer/monomer ratio as an early precipitant of PD.
– Characterize human brain tissue extracted pathological species and their interaction with physiological species.
– Elucidate the mechanism of tetramer assembly in vitro and in cell models.
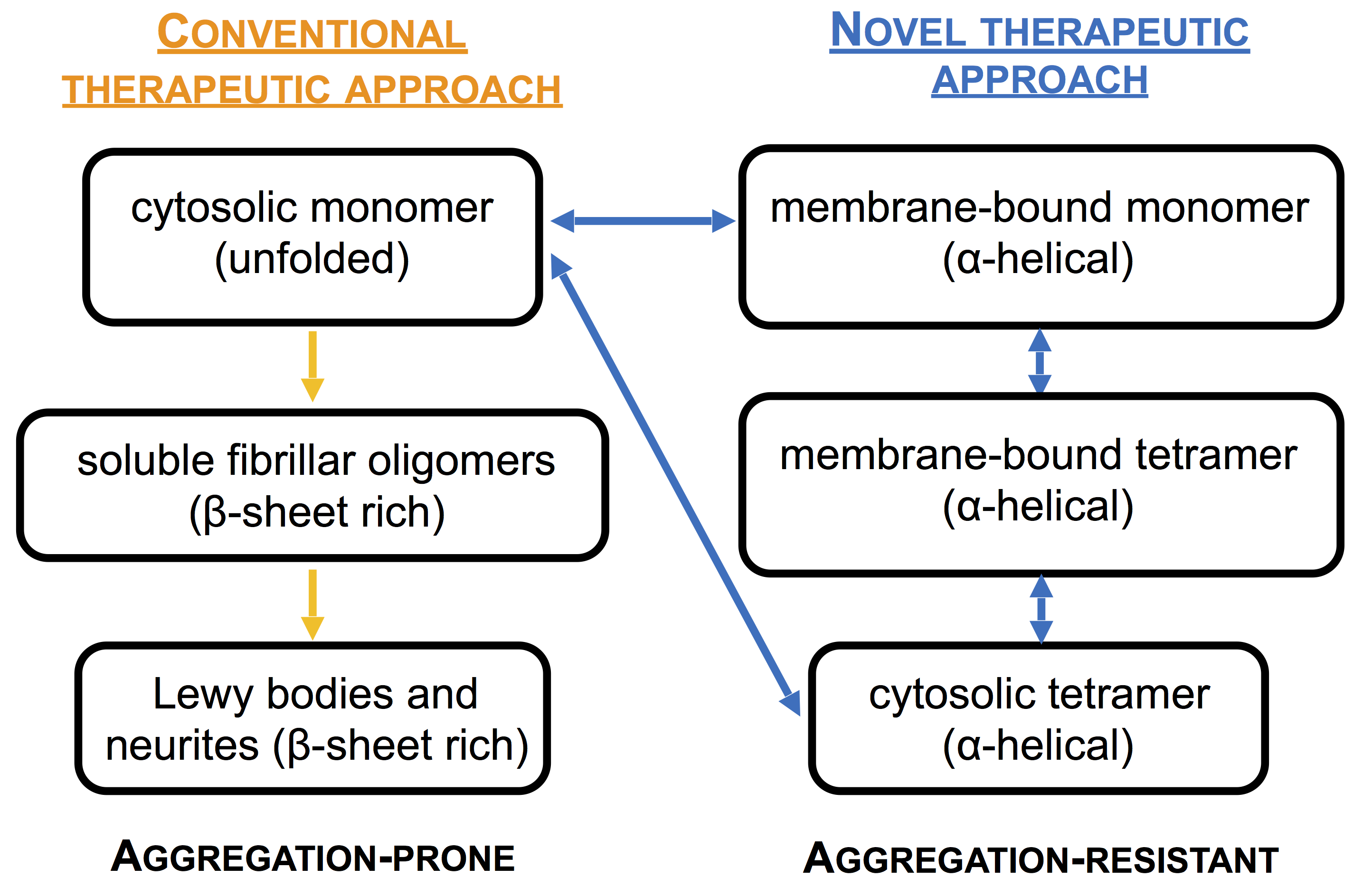
New results from this entirely new concept could point to exciting possibilities in rational PD drug design, based on stabilizing the native tetrameric structure. In addition, quantifying the folding mechanism and the destabilization of physiological tetrameric αSyn to aggregation-prone monomers and subsequent pathogenic αSyn oligomers may be used as a biomarker for diagnosis of synucleinopathies and will provide novel reagents to the community along with valuable platforms for therapeutic compound screening.
